Ne Imf Chemistry
chemistry wallpaperThe are very strong IMFs between molecules of NaCl in the solid. Learn imf chemistry with free interactive flashcards.
Stephens Chemistry Molecule Polarity Imf S And Properties Of Chemical Bonding Schooltube Safe Video Sharing And Management For K12
Then in the last column indicate which member of the pair you would expect to have the higher boiling point.

Ne imf chemistry. Choices for the predominant intermolecular force are metallic bonding ionic bonding network covalent bonding hydrogen bonding dipole-dipole and dispersion forces. In contrast to intra molecular forces such as the covalent bonds that hold atoms together in molecules and polyatomic ions inter molecular forces hold molecules together in a liquid or solid. SI4 or CI4 I chose Sulfur Tetraiodide C.
In order to form a solution the solute must be surrounded or solvated by the solvent. Both enhance the IMF we refer to as hydrogen bonding. When is the total force on each atom attractive and large enough to matter.
This is the only force between 2 nonpolar molecules. Bond Dipoles and Dipole Moments. Hydrogen bonding thats why.
Hydrogen sulfide H 2 S is a polar molecule. Select the Interaction Potential tab and use the default neon atoms. In the following description the term particle will be used to refer to an atom molecule or ion.
Ne Ar Kr Xe d. As was the case for gaseous substances the kinetic molecular theory may be used to explain the behavior of solids and liquids. D diethyl ether CH 3CH 2O.
Note that we will use the popular phrase intermolecular attraction to refer to attractive forces between the particles of a substance regardless of whether these. The molecular weights are H 2 2 CO 28 HF 20. Which of species can exhibit hydrogen bonding among themselves.
LiH HF At which temperature does water have its greatest density. Chemistry Stack Exchange is a question and answer site for scientists academics teachers and students in the field of chemistry. Intermolecular Forces Worksheetpdf 5212 KB.
H 2 Te H 2 Se H 2 O H 2 S b. The attractive forces are stronger for ionic substances than for molecular ones The intermolecular forces of the remaining substances depend on molecular weight polarity and hydrogen bonding. List the types of intermolecular forces that exist between the molecules.
C 3 H 8. CH3CH2OH or CH3CH2Br I choseCH3CH2OH 2. List the substances BaCl 2 H 2 CO HF and Ne in order of increasing boiling points.
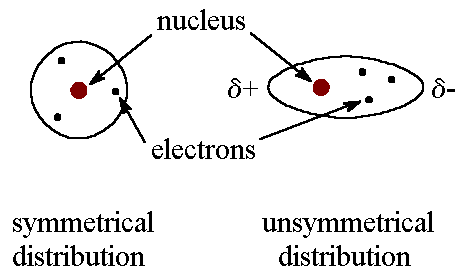
Neon Ne is a noble gas nonpolar and with only modest London Dispersion forces between atoms. Which has the strongest intermolecular forces of attraction. The lighter hydrides have the highest En values which leads to especially polar H-X bonds.
Substance 1Predominant Intermolecular Force. Choose from 500 different sets of imf chemistry flashcards on Quizlet. Dispersion Dipole-Dipole Hydrogen 4 SF4.
C 6 H 6. Select the Total Force button and move the Ne atom as before. Intermolecular forcesattractive and repulsive forces between molecules The strength of the intermolecular forces between solutes and solvents determines the solubility of a given solute in a given solvent.
Then select the Component Forces button and move the Ne atom. Last Modified on November 2 2015 Address 400 W Elm Ave Flagstaff AZ 86001. Section the stronger the IMFs the more E needed to overcome these attractions and vaporize.
Just want to check some answers. Intermolecular forces are generally much weaker than covalent bonds. Move the Ne atom on the right and observe how the potential energy changes.
If you isolate one molecule of NaCl in the crystal structure it is attracted to other NaCl molecules in they solid by ion-ion IMF. HF HCl HBr HI e. Pre-AP Chemistry Lecture Notes.
Ne 25 Ar 95 Kr 125 Xe 170 A Le Châteliers principle D dipole-dipole interaction B hydrogen bonding E none of these C London dispersion forces. The small size of each dipole allows for a closer. The weakest IMF is called London Forces or van der Waals Forces.
Which has the. Therefore Ionic compounds are technically not held together by IMFs. B 2 H 6.
It will be a gas at and well below room temperature boiling at -246C. Polar bonds form between atoms of different electronegativityThis is described as a bond dipole and is represented using an arrow to indicate the direction of. Draw the molecule H 2 O with its correct geometry and show the bond dipoles and partial charges.
This type of IMF clearly is stronger than a H-bond since the attractions are between fully charged ions not partially charged atoms. Draw in the partial charges d- and d on both NF 3 and NH 3. Answer Key IMF Practice test 2 1.
Intermolecular forces are forces of attraction that hold groups of covalently bonded atoms called molecules to other molecules. It only takes a minute to sign up. Note that we will use the popular phrase intermolecular attraction to refer to attractive forces between the particles of a substance regardless of whether these.
0 o C 4 o C 96 o C 100 o C. In the following description the term particle will be used to refer to an atom molecule or ion. As was the case for gaseous substances the kinetic molecular theory may be used to explain the behavior of solids and liquids.
It will have polar interactions as well as London forces between molecules and boils at -60C.
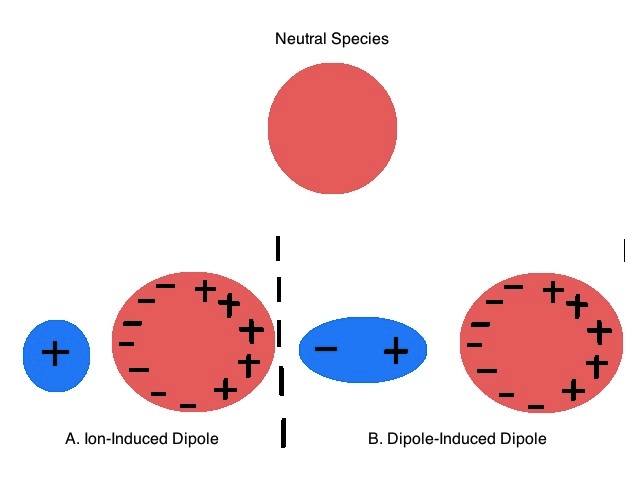 11 4 Nonpolar Molecules And Imf Chemistry Libretexts
11 4 Nonpolar Molecules And Imf Chemistry Libretexts
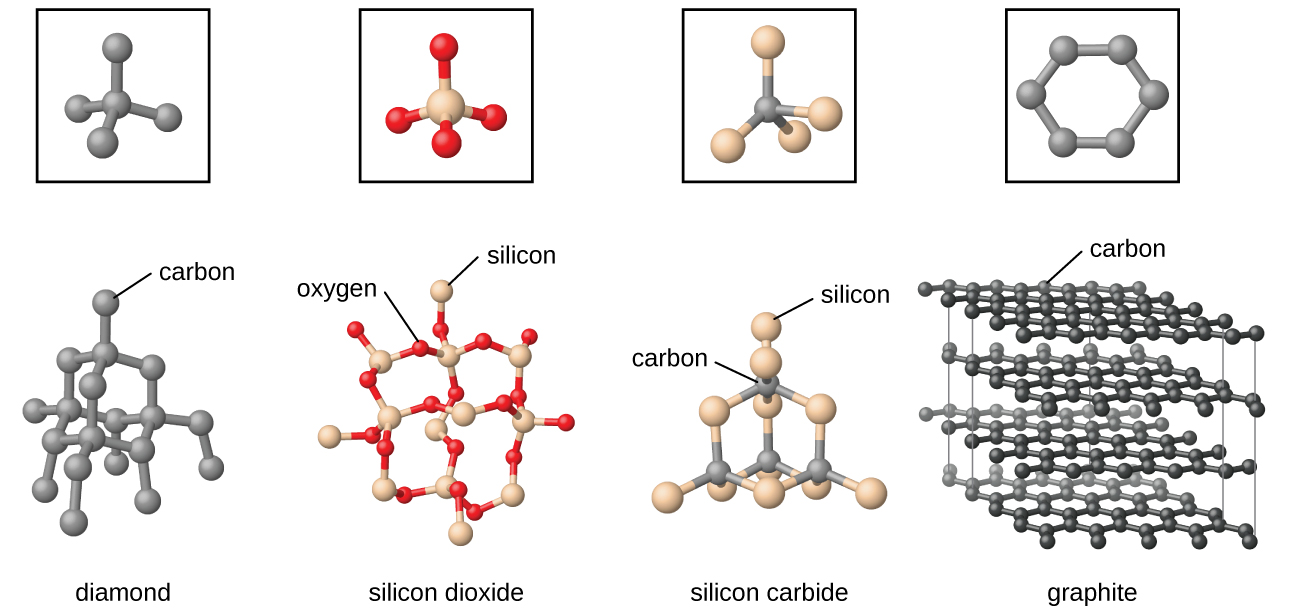 10 5 The Solid State Of Matter Chemistry
10 5 The Solid State Of Matter Chemistry
 Intermolecular Forces Physical Properties Of Organic Compounds Mcc Organic Chemistry
Intermolecular Forces Physical Properties Of Organic Compounds Mcc Organic Chemistry
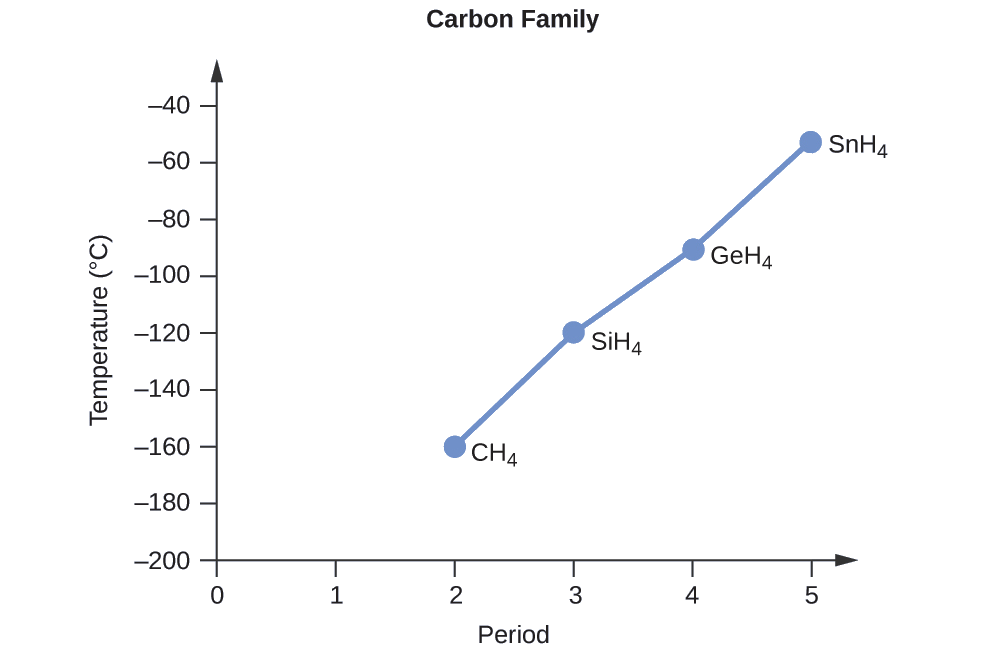 6 3 Forces Between Molecules Chemistry Libretexts
6 3 Forces Between Molecules Chemistry Libretexts
 Ch 10 Intermolecular Forces And Types Of Solids Ap Chemistry Sequoyah High School Ppt Download
Ch 10 Intermolecular Forces And Types Of Solids Ap Chemistry Sequoyah High School Ppt Download
 Pin By Msrazz Chemclass On The Mole Mole Day Chemistry Posters Mole
Pin By Msrazz Chemclass On The Mole Mole Day Chemistry Posters Mole
 Chemistry Intermolecular Forces
Chemistry Intermolecular Forces
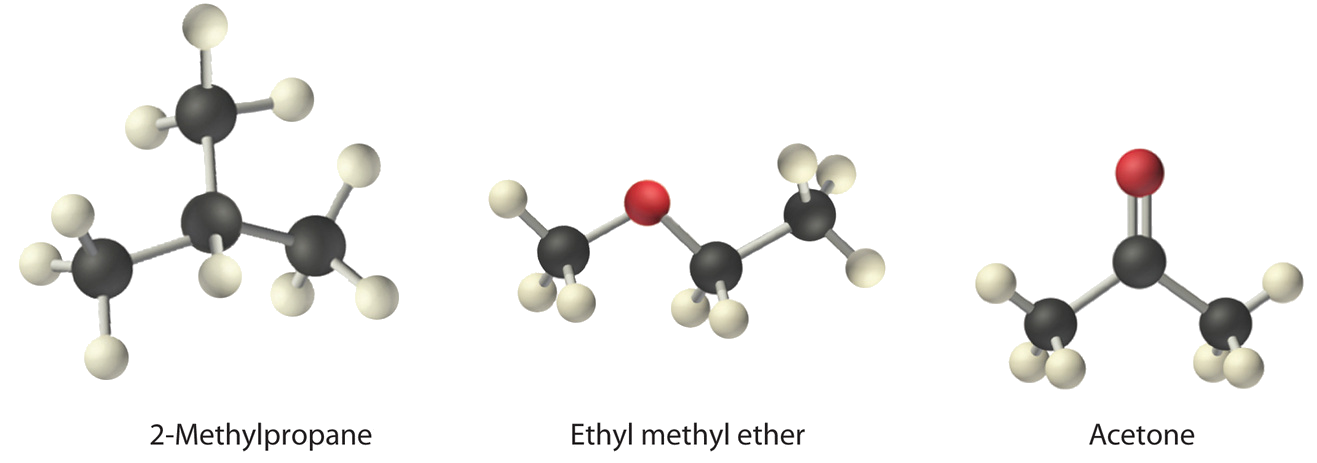 12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts
12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts
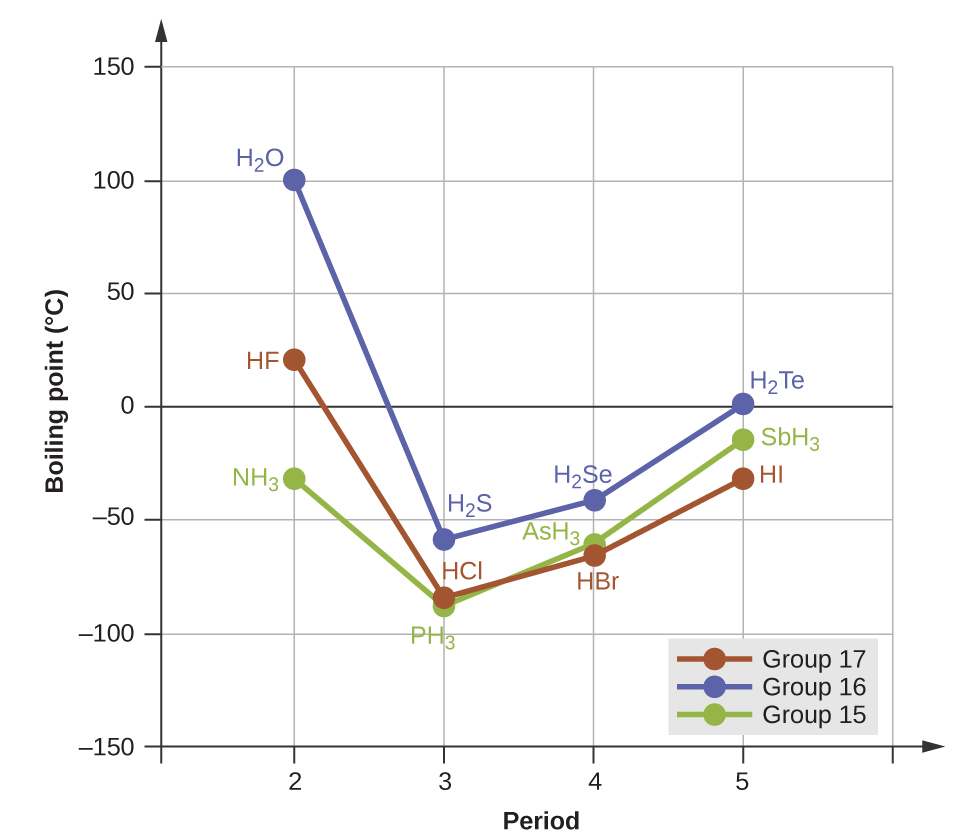 10 1 Intermolecular Forces Chemistry
10 1 Intermolecular Forces Chemistry

 Intermolecular Forces Chemistry Master
Intermolecular Forces Chemistry Master
 Chemistry Puzzle Color By Number Intermolecular Forces Intermolecular Force Chemistry Chemistry Classroom
Chemistry Puzzle Color By Number Intermolecular Forces Intermolecular Force Chemistry Chemistry Classroom
Https Www Studocu Com En Us Document University Of Hawaii At Manoa General Chemistry Ii Lecture Notes Chem 162 Intermolecular Forces Lecture Notes 6142806 View
 Ch104 Chapter 7 Solutions Chemistry
Ch104 Chapter 7 Solutions Chemistry
 Mr Lee Says Atomic Radius Increases As You Go Down A Group Because You Are Adding More Energy Levels Orbit Teaching Chemistry Chemistry Notes Periodic Table
Mr Lee Says Atomic Radius Increases As You Go Down A Group Because You Are Adding More Energy Levels Orbit Teaching Chemistry Chemistry Notes Periodic Table
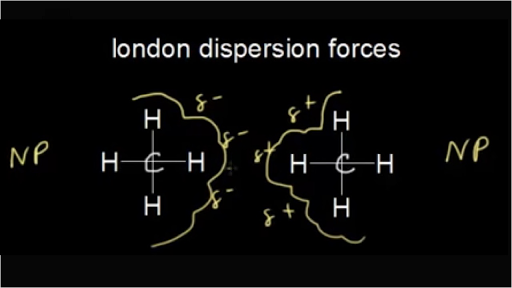
 Physical Chemistry Bonding Flashcards Quizlet
Physical Chemistry Bonding Flashcards Quizlet
 3 7 Intermolecular Forces Chemistry Libretexts
3 7 Intermolecular Forces Chemistry Libretexts